Vaccin dos 3
Information about booster shots for the broader population will be available soon. 3 More recently researchers in Israel reported that.
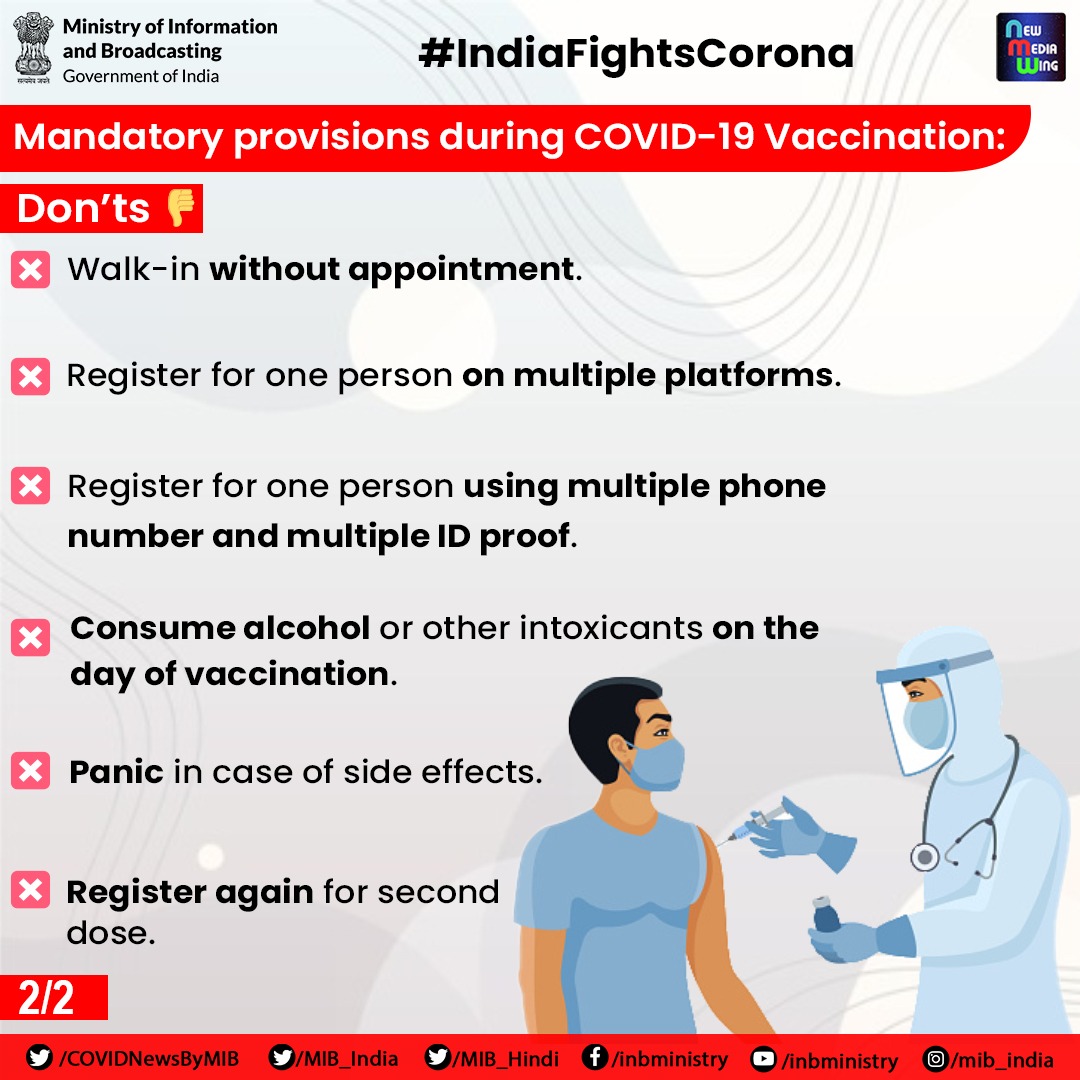
Dos And Donts During Covid Vaccination Vikaspedia
With consideration of the minimum age and interval between doses most routine vaccines can be safely and effectively administered at the same visit.
. 1 or 2 doses. Large-scale Phase 3 clinical trials are in progress or being planned for COVID-19 vaccines in the United States. Concurrent Administration of Vaccines.
3-Dose Vaccine Series for Infants Including the Birth Dose Since 1991 ALL medically stable infants with a birth weight of at least 2000 g in the US. 05 mL primary series. Recommendations from the Australian Technical Advisory Group on Immunisation ATAGI on the use of a third primary dose of COVID-19 vaccine in individuals who are severely immunocompromised.
Refer to Part 4 for vaccine-specific chapters and to Part 3 for condition-specific recommendations. Are recommended to receive the first dose of hepatitis B vaccine within 24 hours of birth. Haemophilus influenzae type b.
Amount of Diluent Needed per Vial. Vaccines in Phase 3 Clinical Trials. La vaccination doit débuter avant lâge de 20 semaines et être terminée avant lâge de 8 mois.
025 mL booster Janssen. Chicken pox varicella 2 doses. Woman 95 totally incapacitated after receiving 3 doses of COVID-19 vaccine COVID-19 reinfection rare but more common in older people study finds Details sparse on.
COVID-19 vaccine clinical trials including vaccines in earlier stages of development by visiting clinicaltrialsgov. This material is provided for educational purposes only and is not intended for medical advice diagnosis or treatment. The additional 2 doses are given at 1 month and 6 months of age.
Version 31 November 12 2021. Some COVID-19 vaccines such as those produced by Pfizer-BioNTech and Moderna require two doses. Measles mumps and rubella.
This document is not intended to provide or take the place of medical advice diagnosis or treatment or legal advice. COVID-19 Vaccine Third Dose Recommendations. Three Covid-19 Vaccine Doses in Transplant Recipients Solid-organ transplant recipients receiving immunosuppressive therapy appear to have a poor.
Fill Volume before dilution 045 mL 13 mL. 1 or more doses. 5 COVID-19 vaccines are currently approved for use in Canada.
4-Dose Vaccine Combination Series for Infants Pentavalent or Hexavalent. To learn more about US. Vaccine Dose Route COVID-19 Pfizer-BioNTech age 5 to 3 mL adultadolescent formulation for primary and booster doses IM.
Doses per Vial. World Health Organization WHO Director-General Tedros Adhanom Ghebreyesus said it was a scandal that six times more booster shots are being. Children ages 5 to 11 may be eligible for the Pfizer-BioNTech Covid vaccine by early next month.
If you do not have a current hepatitis B infection or have not recovered from a past infection then hepatitis B vaccination is an important way to protect yourself. Talk to your health care provider about the right vaccines for you. The recommended schedule for the hepatitis B vaccine.
C 9 months 6 months. 771 billion doses have been administered globally and 2636 million are now administered each day. NOBIVAC INTRA-TRAC 3 INTRANASAL OFFERS ADVANTAGES OVER ORAL AND INJECTABLE VACCINES Dant JC Waszgis B LaFleur RL Xu Z Tarpey I.
In Israel on the other hand unless you received your second dose of the Covid-19 vaccine within the last six months you will now need a third dose to become eligible for a green pass which allows entry to gyms restaurants and other venues. 533 of the world population has received at least one dose of a COVID-19 vaccine. Continue reading The 3-Shot Hepatitis B Vaccine Do I Need to.
Only 5 of people in low-income countries have received at least one dose. HPV human papillomavirus 3 doses. Calendrier pour amorcer la vaccination des enfants âgés de 3 mois à moins de 1 an à la 1 re visite.
Hand holding medical light icon. The report noted a possible link between the second vaccine dose and myocarditis among male patients between the ages of 16 and 30 years. You may need more or fewer vaccines depending on your medical history and risks.
05 mL for primary booster doses Diphtheria Tetanus Pertussis DTaP DT Tdap Td 05 mL. Others such as the Johnson. In Israel on the other hand unless you received your second dose of the Covid-19 vaccine within the last six months you will now need a third dose to become eligible for a green pass which.
Freezer -25C to -15C 2 weeks NA. A complete two-dose COVID-19 vaccine series provides strong protection against COVID-19 infection and severe outcomes including against the Delta variant in the general population. Two shots spaced three weeks apart.
Data sources include IBM Watson Micromedex updated 11 Oct 2021 Cerner Multum updated 1 Nov 2021. Moment propice à la vaccination. Highlights of changes Updated observation period for health care workers page 12 This guidance provides basic information only.
Third doses are being offered to specific high-risk groups to help provide sufficient protection based on a suboptimal or waning immune response to vaccines and increased risk of COVID-19 infection. The Hepatitis B vaccine is a safe and effective 3-shot series that protects against the hepatitis B virus. 03 mL 02 mL.
Refrigerator 2C to 8C 1 month 10. Duration of Immunity for an Oral Bordetella Bronchiseptica Vaccine. ATAGI statement on the use of a 3rd primary dose of COVID-19 vaccine in individuals who are severely immunocompromised.
805am Jul 29 2021. The bottom line. A third dose of the PfizerBioNTech Covid-19 vaccine can strongly boost protection against the Delta variant -- beyond the protection afforded by.
Par la suite poursuivre la vaccination avec le calendrier régulier. The COVISHIELD version of the AstraZeneca vaccine is no longer used in Canada as the interim order for its use expired on September 16 2021. 26 2021 Updated Oct.
La 3 e dose administrée sera un vaccin à ARN messager soit celui de Pfizer ou de Moderna. ULT Freezer - 90⁰C to -60. For Healthcare and Public Health.
The Australian Technical Advisory Group on Immunisation ATAGI have recommended a third dose of COVID-19 vaccines for people who are severely immunocompromised. 18 mL 13 mL. When a person is.
COVID-19 vaccination began in Canada on December 14 2020. Return to table 1 footnote 2 referrer. Pfizer-BioNTech COVID-19 Vaccines.
Based on the. ISCAID Proceedings Portland OR 2018. The National Advisory Committee on Immunizations NACI recommendations on the use of.
La dose de rappel pour les 164 458 Québécois qui ont reçu le sérum à vecteur viral AstraZeneca ou. 3 or 4 doses. But unlike kids.
6 doses per vial after dilution 10 doses per vial after dilution Storage Conditions. Read the statement about third doses of COVID-19 vaccines for people who are severely immunocompromised.
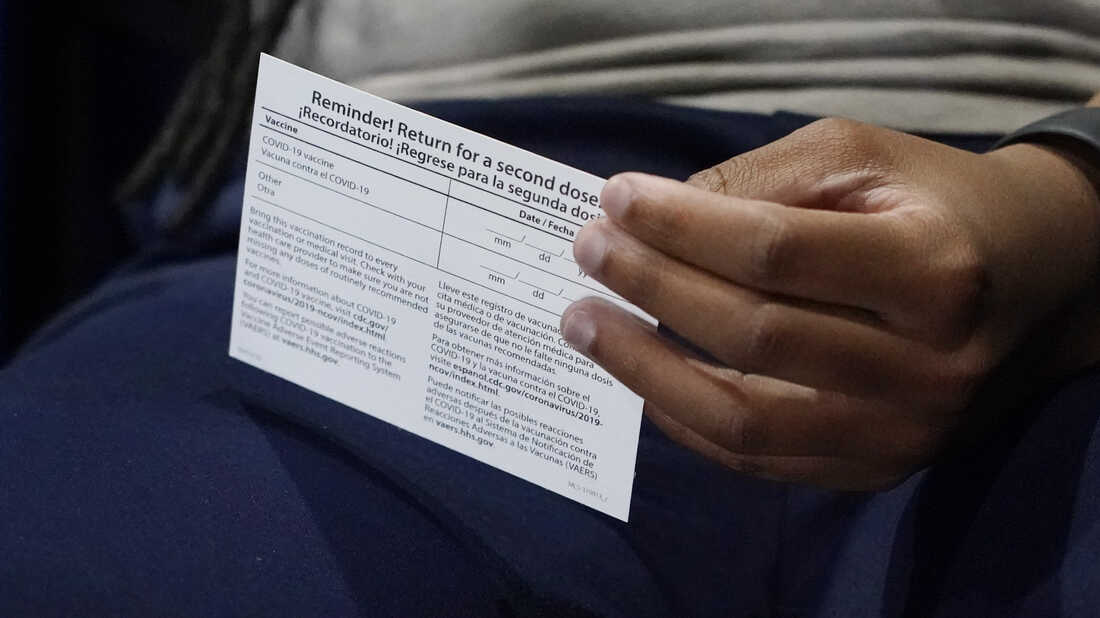
How To Keep The Vaccination Card With You Coronavirus Updates Npr

Vaccine Against Covid 19 I Vastra Gotaland Kry
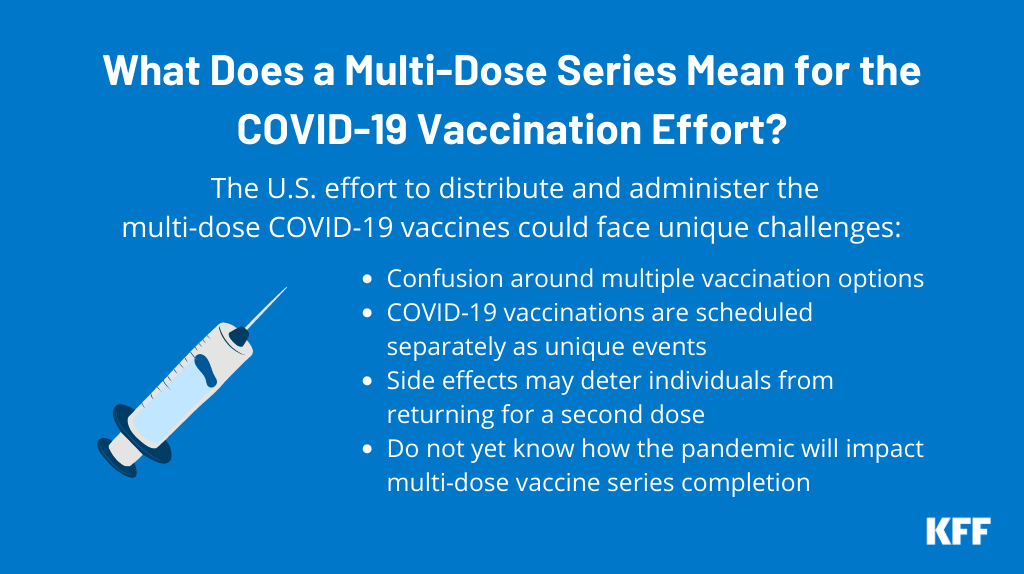
What Does A Multi Dose Series Mean For The Covid 19 Vaccination Effort Kff
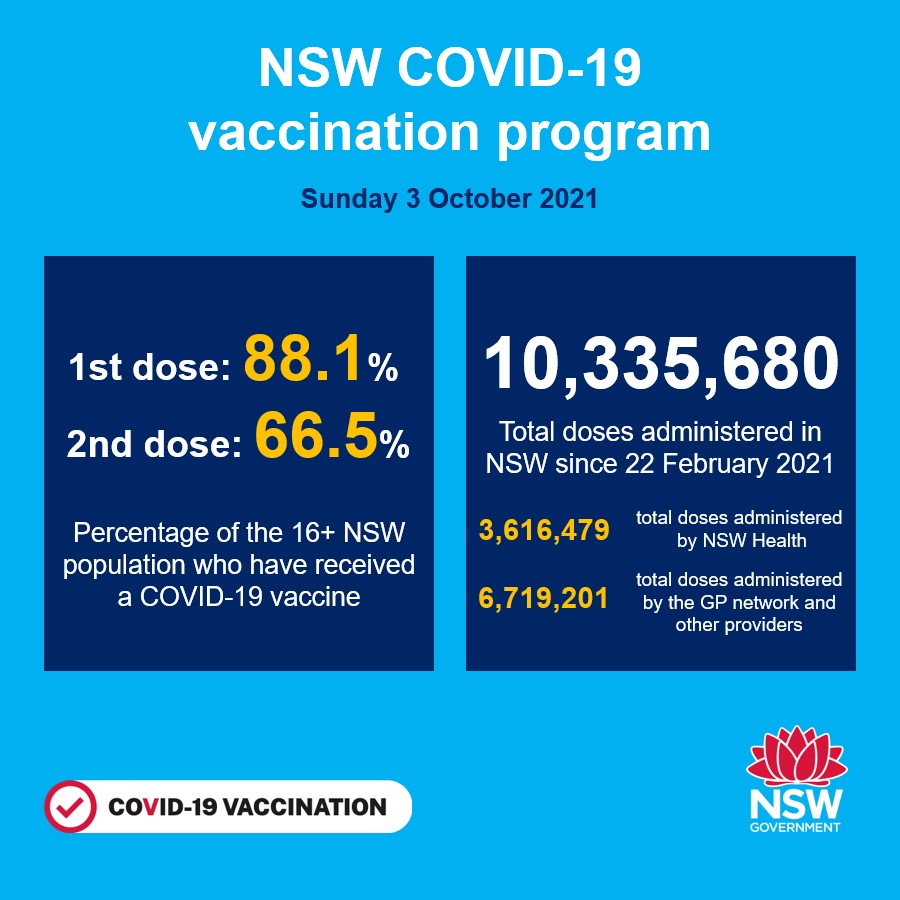
Nsw Health On Twitter Nsw Recorded 667 New Locally Acquired Cases Of Covid 19 In The 24 Hours To 8pm Last Night Https T Co Jotsdr1jq6 Twitter

Human Papillomavirus Vaccination Uptake In Low And Middle Income Countries A Meta Analysis Eclinicalmedicine

You Re Fully Vaccinated Now What Lee Health

Covid 19 Vaccination Drive In India Dos Don Ts And Possible Side Effects Coronavirus Outbreak News
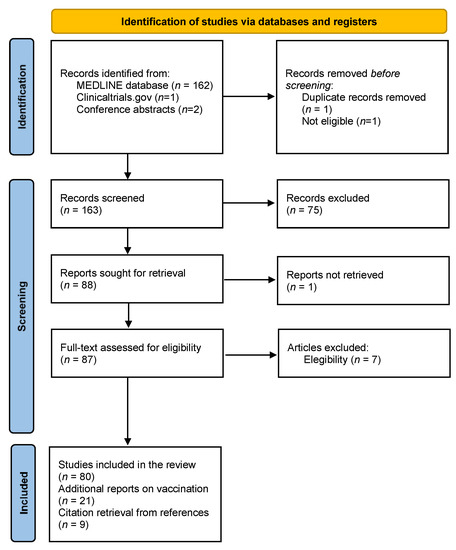
Vaccines Free Full Text Multiple Sclerosis Disease Modifying Therapies And Covid 19 A Systematic Review On Immune Response And Vaccination Recommendations Html

Covid 19 Vaccine Communications Resources National Governors Association

Covid Vaccination Dos And Don Ts To Remember As Vaccines Rollout For All Adults From May 1 News

Covid19 Do S And Don Ts After Vaccination

Dos And Donts During Covid Vaccination Vikaspedia

Los Angeles Oks One Of Strictest Us Vaccination Mandates

Mumbai Covid Vaccination Centres To Be Closed For Next 3 Days Amid Vaccine Shortage
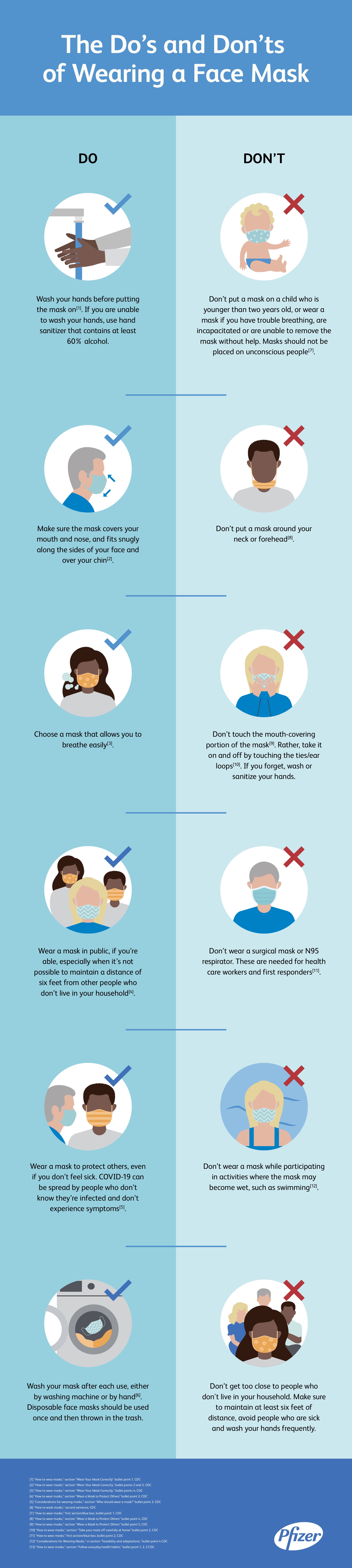
The Do S And Don Ts Of Wearing A Face Mask Pfizer
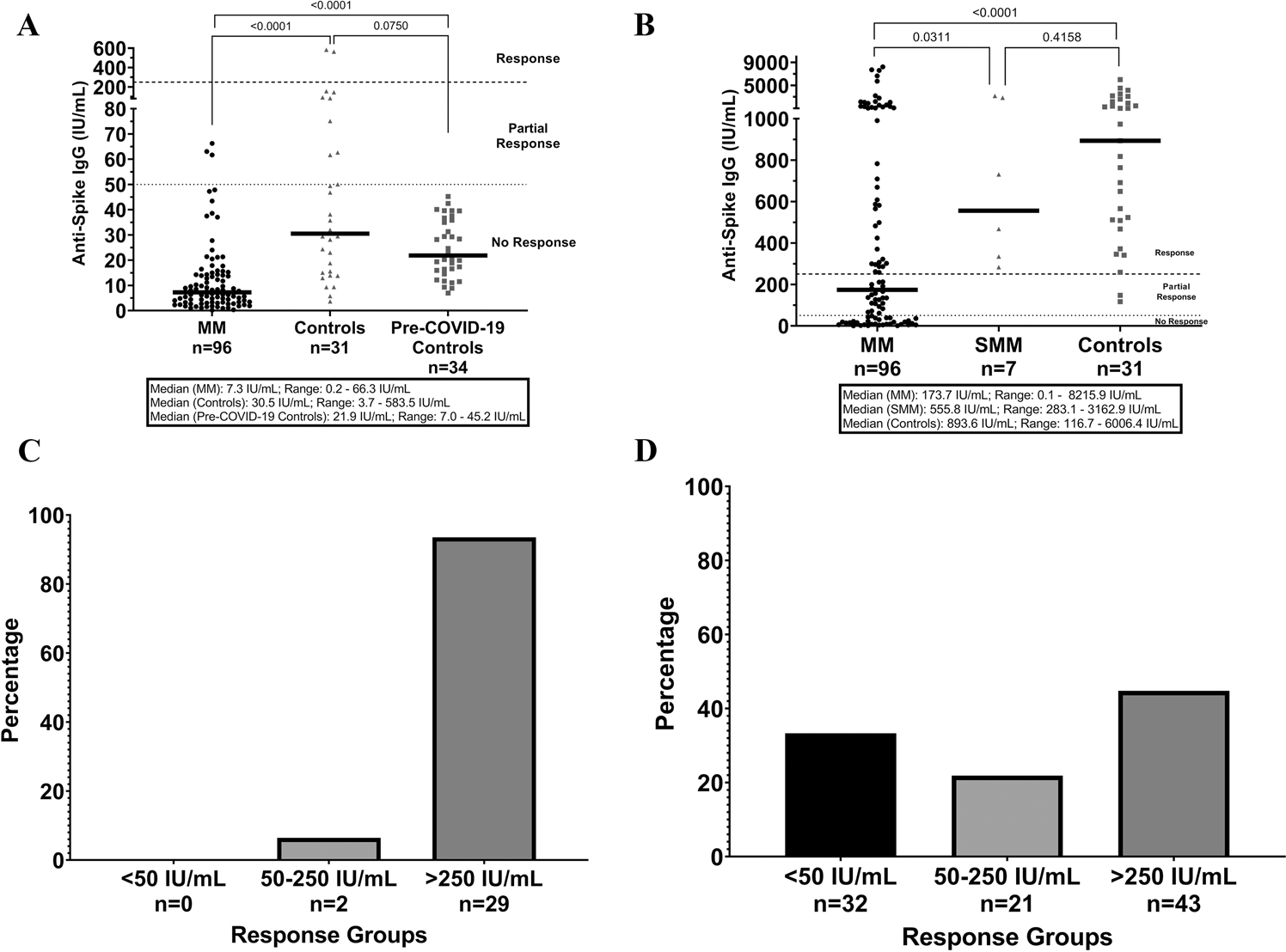
Response To Mrna Vaccination For Covid 19 Among Patients With Multiple Myeloma Leukemia